We are conducting a research study at participating hospitals located across Ontario to measure the short and long-term effects of COVID-19 vaccination in pregnancy. While much is known about COVID-19 vaccination safety in pregnancy, there is less information about the duration of antibodies in pregnant individuals and the transfer of COVID-19 antibodies to their babies and to their breastmilk. This study will help Canadian families make informed decisions related to COVID-19 vaccination.
Why is this study being done?
The purpose of this study is to find out what effects COVID-19 vaccines have on the immune systems of pregnant women/individuals and their babies after they are born. We will measure immune responses in vaccinated participants and their babies, and document vaccine-related reactions and health outcomes that may occur after vaccination.
The findings from this study will provide valuable information to Canadian families, public health officials and health care providers.
What will happen during this study?
If you decide to participate, you will be asked to complete electronic surveys and give biological samples during your pregnancy and after you have delivered. We will also ask to collect samples from your baby once they are born.
All aspects of this study are voluntary. It is okay if you decide to complete just some of the study surveys. It is also okay if you provide some of the study samples. You will be free to stop your participation at any time.
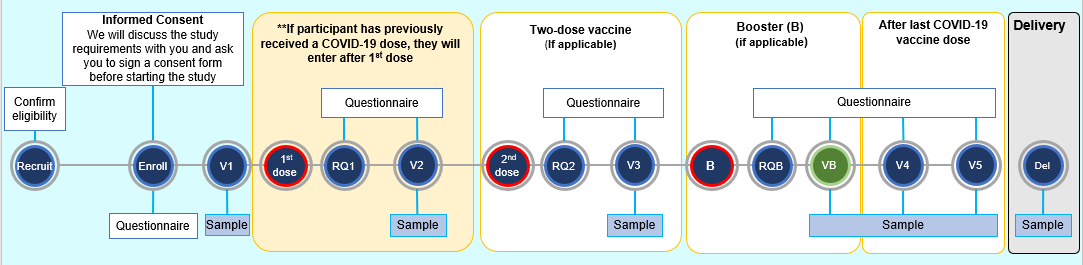
In recognition of your contributions, you will receive a $5 gift card to a coffee shop at each in-person study visit for sample collection. We will also cover your parking expenses and/or bus-fare incurred for any study visit outside of your regularly scheduled clinical visits.
You are eligible if:
However, you are NOT eligible if:
1. You are aware of any major health concerns of your unborn baby; OR
2. You are pregnant due to surrogacy, or planning to give your child up for adoption
If you believe you are eligible to participate in this study, please contact our study team at 613-737-8899 ext. 77295 or you may fill in the “Contact Us” form and our study team will contact you.




Last modified date: March 12, 2024