For more information about the OMNI Research Group and their projects, visit the new site at https://ohri.ca/en/obstetrics-maternal-newborn-investigations-omni
Gestational diabetes mellitus (GDM) is a common pregnancy complication that can lead to various negative health outcomes for both mother/birthing parent and baby. Recent research suggests that induction of labour (procedures or drugs used to stimulate birth) between 37 and 41 weeks of pregnancy may reduce some of these risks compared to expectant management (waiting until labour begins on its own or prompting labour and/or delivery to occur earlier if medically necessary).
However, there is still limited research, and no agreement amongst medical experts on the benefits of inducing labor after 37 weeks in pregnancies affected by GDM.
The purpose of this study, called a pilot study or a feasibility study, is to test if it is reasonable to conduct a larger study to determine if induction of labour between 38 weeks + 0 days and 38 weeks + 6 days gestation reduces the risk of serious health problems among pregnant individuals with GDM and their infants, compared to expectant management.
You might be eligible if:
If you decide to participate, you will be put into a group by chance (like flipping a coin) into one of the groups below.
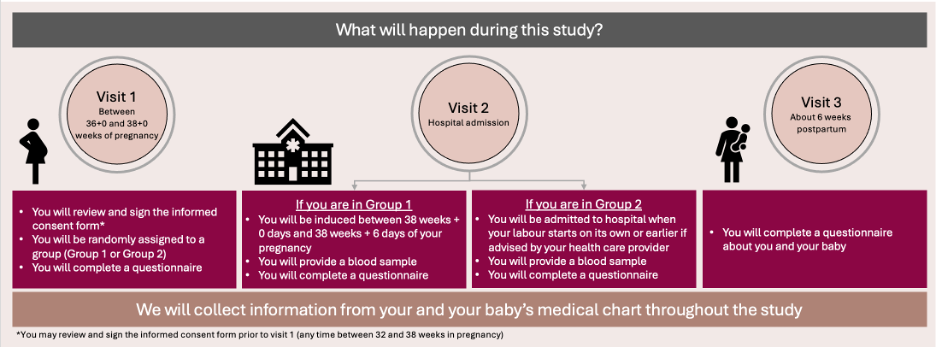
Your participation will last from the day you provide consent to participate until 6 weeks after you are discharged from the hospital after your baby is born. Once you have been put in a group, you will be asked to complete three electronic questionnaires and give a blood sample during your pregnancy. Information about your pregnancy, delivery and newborn will be collected from your medical charts.
You may fill in the “Contact Us” form below and the research team at your planned delivery hospital will contact you.




Last modified date: March 12, 2024